Registration Information
Swiss2Care sp. z o.o. sp.k.
Mostowa 38/1
87-100 Toruń
BDO: 000457104
NIP: 8792720173
KRS 0000830200
The course of a parallel import

Obtaining the permit
A medicinal product subject to parallel import must have a permit issued by the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products.

Repackaging
The imported product is repackaged by entities authorized to do so. The immediate packaging of products (e.g. blisters) remains original, without affecting the original quality of the product.

Distribution
Medicines are stored and distributed under conditions that ensure unchanged properties and quality.
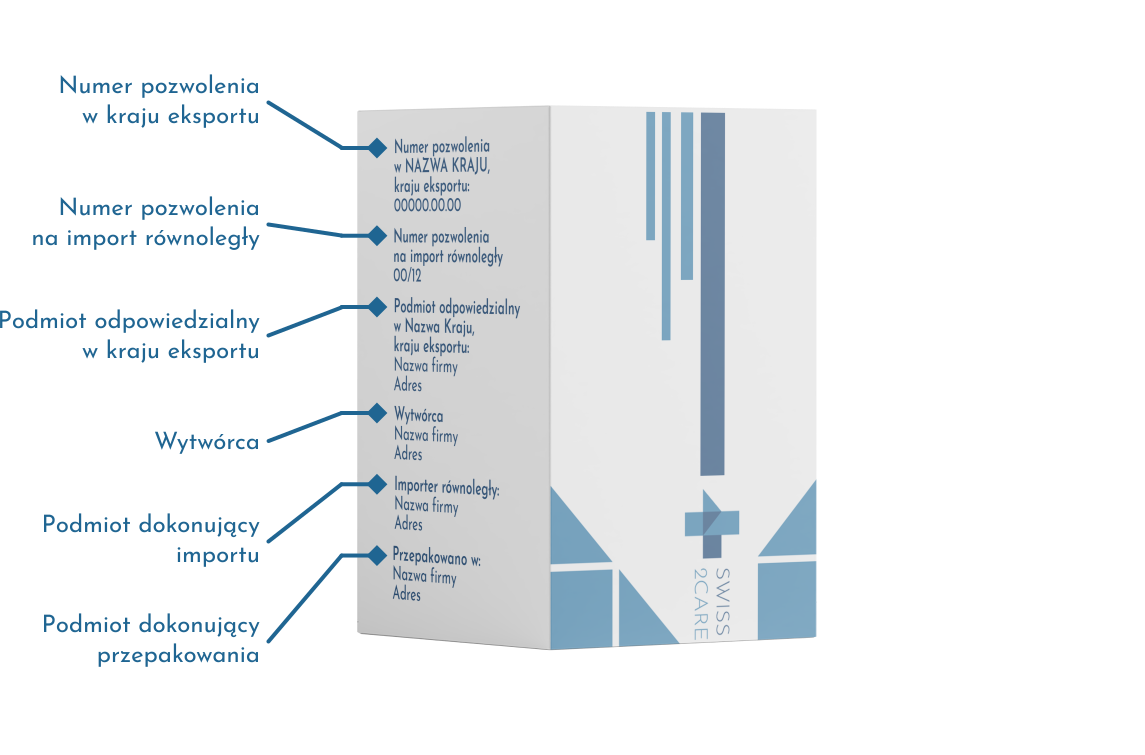
How to identify drug packaging from parallel import?
What are the advantages of such drugs?

Standards
Swiss2Care, as a distributor of such drugs, is subject to a distributor license. We are committed to applying internal supply controls, including product and supplier verification, and to ensuring that the quality and integrity of drugs in the supply chain are maintained.

Safety
Imported drugs are 100% safe. They must have the same active substance, strength, dose, route of administration and form as the medicinal product with a marketing authorization in Poland.

Savings and Balance
By conducting parallel import, we offer a product negotiated directly with suppliers from the European Economic Area, attractive in terms of quality and price, but also counteract shortages of drugs by importing them from markets with a surplus.
